BACKGROUND
Since the FDA's initial indication for the use of daratumumab in relapsed/refractory multiple myeloma (RRMM) in November 2015, usage has been expanded by subsequent approvals in newly diagnosed, transplant-ineligible MM patients, and more recently in newly diagnosed transplant-eligible patients. Real World Data (RWD) has been published demonstrating daratumumab's efficacy in RRMM settings[1]and its utility of split dosing for the initial dose[2]. However, no study has been published to examine the real-world dosing patterns of daratumumab compared to the FDA standard dosing schedule. While there are minimal variations in the approved dosing schedule of daratumumab, generally accepted dosing is weekly in week 1 through week 8, q 2 Wks in week 9 through week 24, and q 4 Wks after week 24, all at the standard 16 mg/kg dosage[3]. In its approved dosing schedule, adjustments are made in the frequency of administration, but not in the standard weight-based dosing.
METHODS
Utilizing the Integra Connect database which contains 17 community oncology network accounts and over 1,900 providers in US, we collected all MM patients treated with daratumumab between January 1, 2016 and March 31, 2020. We then excluded any patient whose first line of therapy (LOT) was ambiguous, in order to correctly identify the daratumumab-containing LOTs. We also excluded LOT 1 daratumumab transplant induction due to the wide variation in daratumumab dosing schedules in clinical trials in the transplant eligible patient[4]. LOTs were determined based on International Myeloma Working Group guidelines[5].
Data were collected on the date of each individual daratumumab administration, counting initial split dose, if utilized, as 1 dose. The duration of daratumumab and number of doses administered were calculated and corrected for any time on treatment breaks. The study was conducted per individual patient by LOT cohorts, and for the entire cohort of patients. We utilized the standard dose schedule for daratumumab noted above to establish the expected doses of daratumumab and calculated the compliance dose ratio (ratio of actual doses to expected doses per time on therapy) to evaluate how closely real-world treatment adhered to the standard dosing schedule.
RESULTS
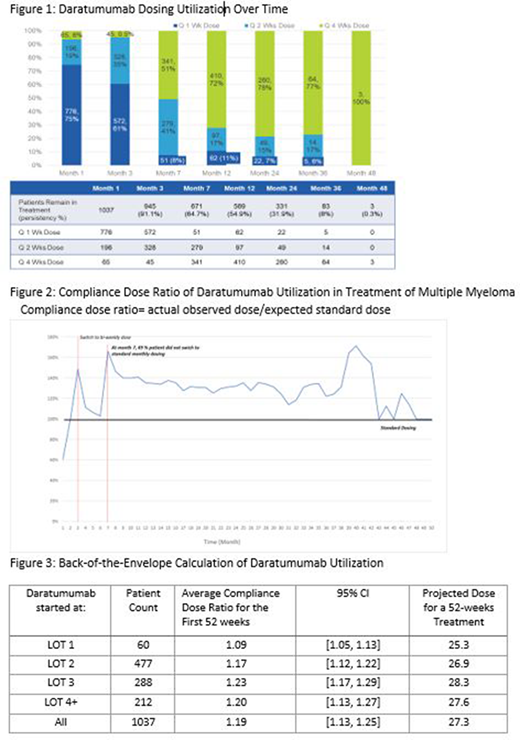
1037 MM patients were included with at least 6 doses of daratumumab administration and without stem cell transplant or uncertain LOT. Across all LOTs, the mean duration of daratumumab treatment was 5.6 months with a median duration of 9.8 months. After week twenty-four, 671 (65%) patients remained on daratumumab-containing regimens, with 330 patients continuing q 1 Wk or q 2 Wks dosing, whereas the standard would employ a switch to q 4 Wks dosing (Figure 1). Overall compliance dose ratio was consistently above 100%, implying a significant proportion of patients were receiving more frequent dosing than expected under the standard dosing schedule (Figure 2). We carefully evaluated patients in various LOTs and combination therapies. Drug combination was not found to exert a significant impact on the daratumumab dosing pattern. Compliance dose ratio of daratumumab is slightly higher in RRMM compared to the dose ratio in LOT 1 newly diagnosed MM, but even LOT 1 has a ratio greater than 1 (Figure 3). It should be noted that this increased compliance dose ratio is present in all LOT cohorts despite 25% of patients being started on doses less frequent than weekly (Figure 1).
CONCLUSIONS
In real-world community oncology practices, daratumumab is utilized in a more frequent dosing schedule than the FDA approved standard dosing. With standard dosing there are 23 daratumumab doses in the first 52 weeks. The compliance dose ratio found in our RWD implies 27.3 doses in the first year for the entire cohort and 26.9 and 28.3 doses in LOTs 2 and 3 respectively. Thus, significantly increased drug and administrative costs are incurred over those anticipated in respect to daratumumab dosing utilization. This study is limited to the EMR and administrative claims data of those individuals who are being treated in a community oncology setting. Residual confounding and bias may exist due to entry error and unobserved patient characteristics.
References
[1] Gergely Varga, et al; Blood 2018; 132:3257
[2] Rifkin R, et al; Clin Ther. 2019;41(5):866-881
[3] DARZALEX® [Prescribing Information]
[4] Abdallah N, et al. Ther Adv Hematol. 2019 Dec 23
[5] Rajkumar SV, et al Blood. 2015;126(7):921-922
Smith:Integra Connect: Current Employment; Sanofi: Research Funding. Xue:Sanofi: Research Funding; Integra Connect: Current Employment. Marks:Sanofi: Research Funding. Scott:Integra Connect: Current Employment; Sanofi: Research Funding. Blanc:Sanofi: Research Funding. Nagovski:Sanofi: Research Funding. Lambert:Sanofi: Research Funding; Integra Connect: Current Employment. Varughese:Integra Connect: Current Employment; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.